There can be an abundance of confusion with water activity instruments concerning test time. Some instruments claim a 5-minute test time while others offer fast or quick modes. The truth is that water activity test time is determined by the sample and not the instrument. Since water activity is an equilibrium measurement, a reading is not complete until vapor equilibrium has been achieved and this process cannot be sped up by an instrument (1). So, any claim to a specific test time is illogical and would only be true for select samples. The reality is that most types of samples require a minimum of 5 minutes or more to reach true equilibrium and test times that are faster than that are either using a prediction or the system uses end-of-test settings that are not stringent enough to achieve true vapor equilibrium.
End of Test Requirements
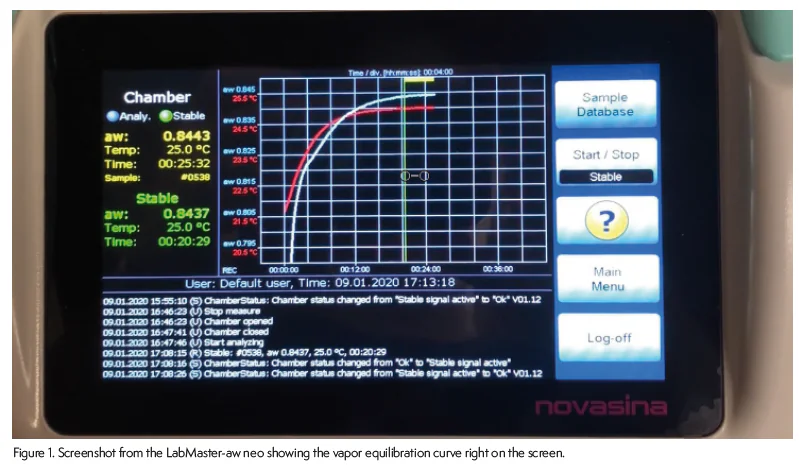
To determine when vapor equilibrium has been achieved and a test should end, instrumentation looks for the rate of water activity change to fall below some setpoint. What setpoint is used and how is it determined will impact both test time and reliability. If the setpoint is set to too low of stringency, the test could end prematurely before true equilibrium has been achieved. On the other hand, setting the setpoint too stringent could result in unnecessarily long test times. The ideal system would allow the user to set the end-of-test setting to whatever best suits their situation and needs. Further, emphasis should be placed on understanding the equilibration process for a particular same type so that educated decisions can be made about what setpoint to use. The most advanced water activity systems will provide an on-screen graph illusting the equilibration process (Figure 1).
Vapor Equilibrium and Test Time
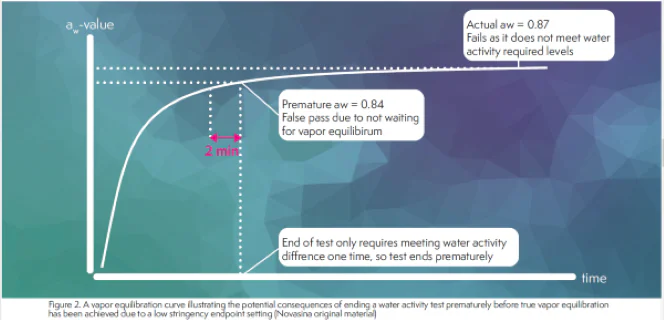
Some water activity instrumentation allows the user to adjust the end-of-test setpoint while others default to a low stringency setting to try to achieve fast test results. These low stringency settings typically only require achieving a preset water activity difference one time and then the test is ended. An instrument with a low stringency setpoint may give a reading in less than 5 minutes, but there is a chance that even though it met the end-of-test requirements, vapor equilibrium had not been achieved. If true, continuing to run the sample without opening the chamber should show a drift until true equilibrium has been achieved. Figure 2 illustrates how this would happen. Water activity changes fast initially, but then starts to level out as vapor equilibrium is achieved, giving a typical equilibrium curve. The first vertical line in Figute 2 indicates where a test that uses low stringency end-of-test requirement might end and give a result. However, notice that if a more stringent setpoint were used, the test would have continued through the equilibrium process, giving a final value that is 0.03 aw higher than the initial reading. Considering that the accuracy of the top water activity instruments is +/- 0.003 aw, a 0.03 aw change is significant. Figure 2 indicates that faster results and higher reliability are mutaully exclusive when it comes to water activity testing.
The vapor equilibrium process is not determined by the instrument or the end-of-test settings, but by the thermal dynamically controlled movement of water from the sample to the headspace. The ambiguity in the end-of-test settings and their potential impact on testing results even lead ISO to define the end-of-test requirements in their recently revised water activity method ISO18787 (2). Dewpoint systems using this ISO18787 setting will not give results as fast as with the default end-of-test settings due to its higher stringency.
How Novasina Handles End-Of-Test Settings
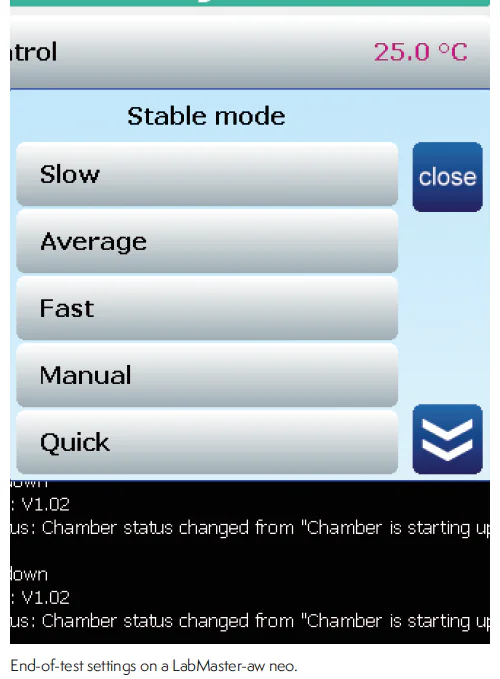
Conversely to defaulting to a low stringency end-of-test setting, Novasina allows the user to set the conditions for ending the test to either more or less stringent. For example, the Slow setting in the Novasina instrument requires no change in water activity greater than 0.001 for 6 minutes and repeated measurements using this setting will not show the drift seen with the other sensors because the setting is stringent enough to ensure true vapor equilibrium. The more stringent settings of the Novasina instrument can result in longer test times, but they also provide a true water activity measurement. The various end-of-test setting from Novasina instruments include:
- Slow – Most stringent – No difference > 0.001 for 6 minutes
- Average – Less stringent – No difference > 0.001 for 4 minutes
- Fast – Least stringent – No diference > 0.001 for 2 minutes
- Manual – Set the stability time – For example, setting to 3 means to run until no difference > 0.001 for 3 minutes
- Quick – Test ends at 10 mins – useful for intermediate checks
- ISO18787 – Uses ISO18787 specified end-of-test requirements
Comparison Testing on Real Samples
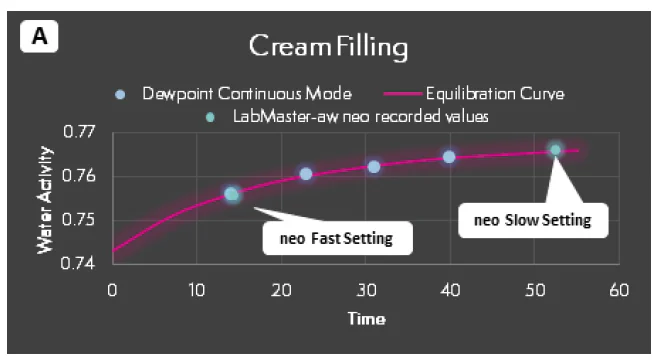
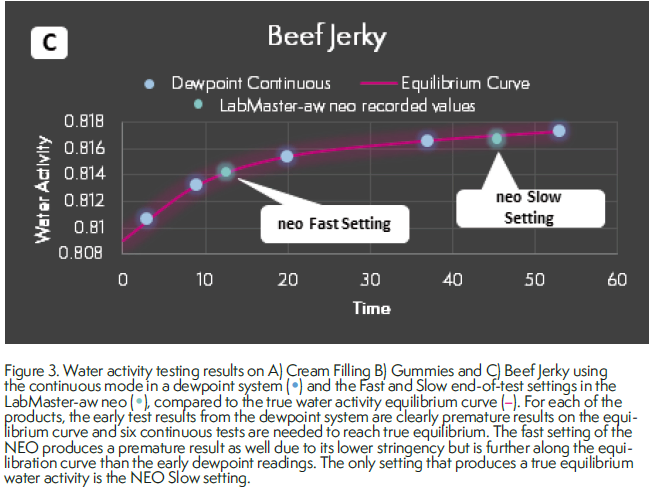
To illustrate the potential problems introduced by prematurely ending water activity tests before vapor equilibrium has been achieved, water activity tests were run on samples of cream filling, gummies, and beef jerky.
Subsamples taken from the same sample were run in a dewpoint instrument in continuous mode and in the Novasina LabMaster-aw neo with the stability set to Fast (see aboive).
The stable results producted by the neo were recorded, but the sample was left in the instrument which allowed the water activity to conrinue to be tracked and graphed on the screen. When the change in water activity was low enough to trigger the end of the test if the stability setting was slow, the test was stopped, and the water activity value recorded.
In the dewpoint instrument, the water activity of each completed test in continuous mode was collected until a result differed from the previous test by less than 0.002 aw.
Figure 3 provides an illustration of the results. the first test result from the dewpoint system was always faster than the test result using the fast stability setting. However, the difference between the first and second result in the dewpoint instrument was always greater than 0.003 aw reported accuracy of the instrument for all products, indicating the first result was premature.
Further, the graphs clearly indicate that the continuous tests are tracking the equilibration curve, which suggests that the first 2-3 test results were premature and vapor equilibrium has not been achived. Alternatively, the slow stability setting on the neo, though it did require test times up to 60 minutes, fully reflected the true vapor equilibrium.

Conclusion
The results presented in this paper clearly show that true vapor equilibrium can require substantial time to achieve, and that the end-of-test settings that are less stringent will give premature results that do not reflect the true water acivity.
If the initial water activity readings from the dewpoint system had been reported, they would have been incorrectly low by as much as 0.02 aw. For a product being made close to the cutoff for microbial growth, that difference could result in releasing unsafe product.
The results further verify that neither the instrument, sensor, or end-of-test settings determine the time needed to reach vapor equilibrium. It is understandable that 30-40 minute test times can be frustrating and there may be justification for using less stringent end-of-test settings for routine testing to improve test times, but this should never been done without first checking on how different these premature results will be from the true water acitvity.
The Novasina water activity instruments are ideally suited to facilitate this because they allow the user to
- adjust the end-of-test settings instead of defaulting to the least stringent setting to disingenuously appear to give fast test results and
- continue to track the results after the test has completed to see how different the premature fast result will be from the true water activity.
Then, an informed decision can be made on whether faster test times are acceptable without putting the company at risk for recalling failed product.
References:
- Fontana, A.J. and Carter, B.P. 2020. Measurement of water activity, moisture sorption isotherms, and moisture content of foods. In Water Activity in Foods: Fundamentals and Applications, 2nd Edition. Wiley-Blackwell.
- International Organization for Standardization. 2017. Foodstuffs – Determination of water activity. ISO18787:2017. https://www.iso.org/obp/ui/fr/#iso:std:63379
The Author
Dr. Brady Carter is a Senior Research Scientist with Carter Scientific Solutions. He specializes in Water Activity and Moisture Sorption applications. Dr. Carter earned his Ph.D. and M.S Degree in Food Engineering and Crop Science from Washington State University and a B.A. Degree in Botany from Weber State University. He has 20 years of experience in research and development and prior to starting his own company, he held positions at Decagon Devices and Washington State University. Dr. Carter currently provides contract scientific support to Novasina AG and Neutec Group. He has been the instructor for water activity seminars in over 23 different countries and has provided onsite water activity training for companies around the world. He has authored over 20 white papers on water activity, moisture sorption isotherms, and complete moisture analysis. He has participated in hundreds of extension presentations and has given talks at numerous scientific conferences. He developed the shelflife simplified paradigm and hygrothermal time shelf model.