By Dr. Brady Carter
Pet food production is one of the fasted growing industries worldwide valued at $91 billion dollars in 2018 (1 ). Gone are the days of feeding your pet scraps from the table; pet food today is carefully formulated to provide healthy diets, avoid allergies, and provide variety. With the increasing value of pet food comes increased expectations for safety, quality, and consistency. Correspondent with increased expectations has been more intensive governmental oversight and regulations. When the Food Safety Modernization Act (FSMA) was signed back in 2011 , it included recommendations to make regulations on pet food production equivalent with human food production. The last of these updates in regulations went in to place for very small businesses as of September 2018 (2). Meeting these new FSMA regulations while still making a safe, quality product that is profitable is the current goal and challenge of the pet food industry.
The foundation of the FSMA approach to food safety is the utilization of Hazard Analysis and Risk-Based Preventive Controls (HARPC) programs. The purpose of these programs is to assess risk of food safety related hazard, implement controls for these hazards, verify that the controls are working, and take corrective actions if they are not (3). The hazards associated with pet food are primarily focused on microbial spoilage by foodborne pathogens, but other hazards such as contamination by metals, chemicals, or adulterants are also targeted. Controls used to prevent hazards depend on the hazard, but for microbial spoilage, the main controls are lethality treatments to kill pathogens and product formulation to prevent microbial growth during storage. These controls must then be monitored through testing such as internal temperature during lethality treatment or water activity of finished product, and the monitoring methods must be verified. Meeting FSMA requirements can be challenging but is made easier by the availability of good instrumentation such as water activity instruments that are reliable and easily verified.
Water activity control has been used as an effective preventative control for decades in the food industry and has been an integral part of the governmental definition of time/temperature control for food safety (4). Although this definition is just now being made equivalent for pet food, water activity control has been utilized in pet food manufacturing since the 1960’ s. “ Gainesburgers”, produced by General Foods Corp, was an intermediate moisture dogfood that had the texture of a wet dog food, but was shelf stable. This was accomplished through formulation using humectants to lower water activity without removing water and was the precursor to the intermediate moisture foods we enjoy today.
While meeting the food safety regulations implemented in the pet food industry by FSMA are certainly critical to the success of pet food manufacturers, there are ways that a product can fail even though it meets all FSMA requirements. This is typically due to quality factors that render the product unacceptable rather than safety concerns. Examples would include chemical changes such as rancidity or physical changes such as texture and appearance. Water activity, along with temperature, is the most important determining factor for the rate of quality loss and shelf life can be maximized by identifying the ideal water activity range for a pet food product. The objective of this document is to provide evidence for why water activity is the most important test that can be performed on pet food by describing how it can be used to both monitor preventative controls for food safety and reduce the rate of chemical and physical breakdown.
Theory of Water Activity
Water activity is defined as the energy status of water in a system and is rootedin the fundamental laws of thermodynamics through Gibb’ s free energy equation. It represents the relative chemical potential energy of water as dictated by the surface, colligative, and capillary interactions in a matrix. Practically, it is measured as the partial vapor pressure of water in a headspace that is at equilibrium with the sample, divided by the saturated vapor pressure of water at the same temperature. The water activity covers a range of 0 for bone dry conditions up to a water activity 1.00 for pure water, resulting from the partial pressure and the saturated pressure being equal. Water activity is often referred to as the ‘ free water’ and while useful when referring to higher energy, it is incorrect since ‘ free’ is not scientifically defined and is interpreted differently depending on the context. As a result, the concept of free water can cause confusion between the physical binding of water, a quantitative measurement, and the chemical binding of water to lower energy, a qualitative measurement. Rather than a water activity of 0. 50 indicating 50% free water, it more correctly indicates that the water in the product has 50% of the energy that pure water would have in the same situation. The lower the water activity then, the less the water in the system behaves like pure water.
Water activity is measured by equilibrating the liquid phase water in the sample with the vapor phase water in the headspace of a closed chamber and measuring the Equilibrium Relative Humidity (ERH) in the headspace using a sensor. The relative humidity can be determined using an resistive electrolytic sensor, a chilled mirror sensor, or a capacitive hyroscopic polymer sensor. Instruments from Novasina, like the Labmaster NEO, utilize an electrolytic sensor to determine the ERH inside a sealed chamber containing the sample. Changes in ERH are tracked by changes in the electrical resistance of the electrolyte sensor. The advantage of this approach is that it is very stable and resistant to inaccurate readings due to contamination, a particular weakness of the chilled mirror sensor. The resistive electrolytic sensor can achieve the highest level of accuracy and precision with no maintenance and infrequent calibration.
While water activity is an intensive property that provides the energy of the water in a system, moisture content is an extensive property that determines the amount of moisture in a product. Water activity and moisture content, while related, are not the same measurement. Moisture content is typically determined through loss-on-drying as the difference in weight between a wet and dried sample. While useful as a measurement of purity and a standard of identity, as this paper will describe, moisture content does not correlate as well as water activity with microbial growth, chemical stability, or physical stability. Water activity and moisture content are related through the moisture sorption isotherm. Table 1 shows that different pet food products can have similar water activities, but very different moisture contents. Obviously, the moisture content associated with a safe water activity will be different for each product and as will be demonstrated in the next section, should never be relied on as an indicator of microbial safety.
| Product | Water Activity | Moisture Content (%d.b.) | |
| Moist Canned Pet Food | 0.994 | 79.6 | |
| Pepared Meals Pet Food | 0.830 | 24.0 | |
| Imitation Bone Treat | 0.679 | 8.43 | |
| Imitation Bacon Treat | 0.669 | 13.0 | |
| Dry Kibbles Dog Food | 0.493 | 8.59 | |
| Dry Kibbles Cat Food | 0.459 | 7.79 |
Microbial Growth
Each microorganism has an ideal water activity inside their membrane and their ability to reproduce and grow depends on maintaining that water activity. When a microorganism encounters an environment where the water activity is lower than their internal water activity, they experience osmotic stress and begin to lose water to the environment since water moves from high water activity (energy) to low water activity. This loss of water reduces turgor pressure and retards normal metabolic activity. To continue reproducing, the organism must lower its internal water activity below that of the environment. It tries to achieve this by concentrating solutes internally. The ability to reduce its internal water activity using these strategies is unique to each organism. Consequently, each microorganism has a unique limiting water activity below which they cannot grow (5, 6). Notice that an organism’ s ability to reproduce and grow does not depend on how much water is in its environment (moisture content), only on the energy of the water (water activity) and whether it can access that water for growth.
A list of the water activity lower limits for growth of common spoilage organisms can be found in Table 2. These growth limits indicate that all pathogenic bacteria stop growing at water activities less than 0. 87 while the growth of common spoilage yeasts and molds stops at 0. 70 aw, which is known as the practical limit. Only xerophilic and osmophylic organisms can grow below 0. 70 aw and all microbial growth stops at water activities less than 0. 60. In addition, microbial growth rate can be modeled using water activity, along with other growth factors such as temperature and pH. For a pet food product to be considered shelf stable, it’ s water activity must be less than 0. 86 aw to ensure that no pathogenic bacteria will be able to grow on the product as it sits on the shelf. Pet food with a water activity higher than 0. 70 aw but less than 0. 86 aw is considered shelf stable but will still support the growth of mold and yeast. Pet products in this range are not considered unsafe because while possibly undesirable to the consumer, molds and yeasts do not cause foodborne illnesses. However, even the growth of non-pathogenic organisms is usually considered to have ended the shelf life of the product. Consequently, the water activity must be reduced to below 0. 70 aw or other interventions such as a preservative system or vacuum packing must be used to prevent mold growth.
| Microorganism | aw limit | Microorganism | aw limit |
| Clostridium botulinum E | 0.97 | Penicillum expansum | 0.83 |
| Pseudomonas feurescans | 0.97 | Penicillum islandicum | 0.83 |
| Escherichia coli | 0.95 | Debarymoces hansenii | 0.83 |
| Clostridium perfringens | 0.95 | Aspergillus femigatus | 0.82 |
| Salmonella spp. | 0.95 | Penicillum cyclopium | 0.81 |
| Clostridium btulinum A B | 0.94 | Saccharomyces bailii | 0.80 |
| Vibrio prahaemoliticus | 0.94 | Penicillum martensii | 0.79 |
| Bacillus cereus | 0.93 | Aspergillus niger | 0.77 |
| Rhizopus nigricans | 0.93 | Aspergillus ochraceous | 0.77 |
| Listeria monocytogenes | 0.92 | Aspergillus restrictus | 0.75 |
| Bacillus subtilis | 0.91 | Aspergillus candidus | 0.75 |
| Staphylococcus aureus (anaerobic) | 0.90 | Eurotium chevalieri | 0.71 |
| Saccharomyces cereviciae | 0.90 | Eurotium amstelodami | 0.70 |
| Candida | 0.88 | Zygosaccharomyces rouxii | 0.62 |
| Staphylococcus aureus (aerobic) | 0.86 | Monascus bisporus | 0.61 |
Chemical Stability
The water activity of intermediate moisture and dry pet food will typically be less than 0. 70 aw, indicating that microbial growth is not likely to occur. However, pet foods in this range do not have unlimited shelf life. So what other modes of failure are likely to occur to end shelf life. For pet food in the 0. 40-0. 70 aw range, chemical degradation is a strong candidate because reactions rates are at a maximum. Chemical reactions such as Maillard browning, lipid oxidation, enzymatic, etc. can affect the taste, appearance, and nutritional value of pet food products. Water activity influences reaction rates by reducing activation energy, increasing mobility, and increasing the rate constant. Consequently, reaction rates are better correlated to water activity than moisture content. In general, as water activity increases so do reaction rates, but specific correlations depend on the type of product and the reaction (Figure 1 ). Most reactions will reach a maximum in the range of 0. 70-0. 80 aw due to dilution at high water activities, but lipid oxidation is the only reaction that increases at low water activity.
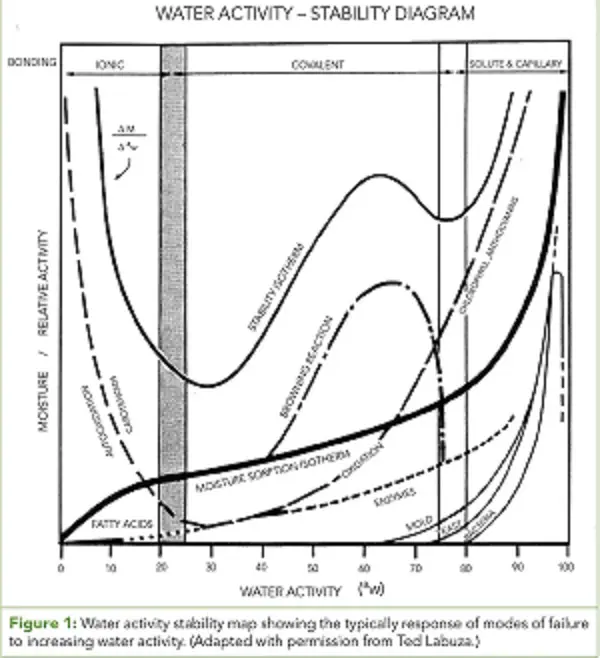
The reaction that is most likely to impact the quality of pet food is lipid oxidation or rancidity. This is a complex reaction with multiple possible pathways and requires the presence of lipids (fat), oxygen, and free radicals to occur. Consequently, it is most often controlled through the removal of oxygen by nitrogen flush or the use of oxygen absorbers. Rancidity occurs when lipid oxidation results in the formation of odor compounds that result in a musty smell and taste. Dry pet food is often sprayed with a fat coating to maintain freshness and improve nutrition, making them particularly susceptible to rancidity. Pets will often reject food that has experienced rancidity, or the owner will discard food that smells rancid. As stated earlier, lipid oxidation is unique in that its rate not only increases as water activity increases, but it also increases at low water activity making the general rule that lower water activity is better not true in all cases.
To aide in determining the ideal water activity for slowing down chemical degradation, the reaction rate can be predicted using shelf life models. To be effective, these models need to account for the effect of water activity and temperature. The only fundamental shelf life model that includes both water activity and temperature is hygrothermal time (7). It is derived from a form of the Eyring (8) equation for rate change and Gibbs equation for free energy and is given by
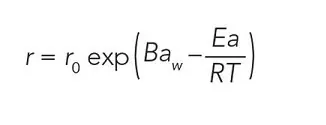
Where T is the termperature (K), R is the gas constant (J mol-1 K-1 ), Ea is the activation energy (J mol-1 ), B is the molecular volume ratio, aw is the water activity, and r0 is the rate at the standard state. In practice, the values for B, Ea/R and r0 will be unique to each situation and are derived empirically through least squares iteration. Once the constants are known, any temperature and water activity can be used with the hygrothermal time model to determine rate of change at those conditions and hence the shelf life for a particular product, as it relates to that change.
Physical Stability
For low water activity (0. 20-0. 40 aw) pet food such as dry kibbles, chemical reactions and microbial contamination are not the most likely mode of failures. However, these products also do not have unlimited shelf life. The most likely mode of failure for these dry products is a change in product texture. Changes in water activity can affect both structure and texture and each product has an ideal water activity range where the texture will be optimal. To maximize shelf life, a product must be manufactured to its ideal water activity range and remain at that water activity during transport and storage. For dry kibbles, water activity is low, and the expected texture is crisp and crunchy but if the water activity increases outside of the ideal range, the kibbles will become soft and undesirable. On the other hand, semi-moist pet treats have higher water activity values and are expected to have a soft and pliable structure. I f the water activity decreases outside the ideal range in semi-moist products, they will become hard and undesirable.
Investigations have shown documented changes in crispiness when equilibrating crisp products to various water activity values. (9, 10). Using both sensory panel data and instrumentation, the relationship between crispness, as sensed by the panel, and water activity was essentially linear, allowing identification of a water activity range where crispness changed from acceptable to unacceptable. In general, crisp products will maintain their texture until they move beyond the critical water activity where a sigmoidal loss in texture will occur (Figure 2).
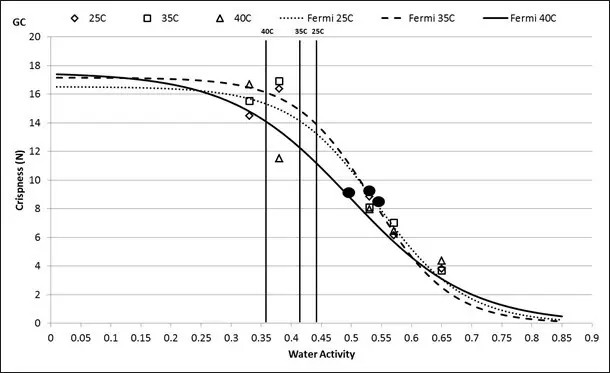
One way that the water activity of dry pet food can change is due to moisture migration. In a package of pet food, water will move between pieces of pet food, if water activity levels differ, regardless of the moisture content of the pieces. Water moves from high water activity (energy) to low water activity and not from high to low water concentration. I f soft and hard pieces are combined at different water activities, moisture migration will occur and could result in texture changes for each of the components. To avoid this problem, the hard and soft pieces must be designed to be the same water activity. I f components are combined at different water activity levels, a model can be used to predict the final equilibrium water activity.
The other way that the water activity of dry pet food could change and result in undesirable changes in texture is due to exposure to high room humidity. As described in the theory section earlier, water activity is also the equilibrium relative humidity and related to the storage humidity. I f a product with a water activity of 0. 40 aw is exposed to a storage relative humidity of 60%, the product will absorb water from the environment until its water activity is equilibrated 0. 60 aw. This process of course takes time, but if not protected, the water activity of the product will increase outside the ideal range and become soft. Placing the product in moisture barrier packaging will slow down the change in water activity. The rate of water activity change inside a package of known moisture permeability can be modeled using fickian diffusion, as can the required package permeability to achieve a desired shelf life (11 ).
Ingredient Inspection
Once an ideal water activity specification has been identified, the next challenge will be to consistently produce product at that ideal level. Ideally, production settings such as oven temperature and belt speed could be established and remain the same through each production run, producing product with the same water activity each time. Unfortunately, there are outside factors that necessitate adjustments to production settings. These factors include, but are not limited to, inconsistency in the incoming ingredients and changes in the production environment. I f production settings are ideally set to achieve the desired water activity under the assumption that incoming ingredients will have a certain water activity, deviation from that expected water activity will produce product of varying water activity. Typically, this is not known until the first product is completed and reaches QA testing. However, at this point, if the product doesn’ t meet specification, it must be re-worked or becomes waste.
An effective solution to avoid problems caused by inconsistent incoming ingredients would be to track the water activity of the ingredients and establish an acceptable range that will produce product meeting specifications with a limited amount of production adjustment. This could be easily accomplished by obtaining a subsample of incoming ingredients and conducting a water activity test. I f the test does not meet the water activity requirement for the ingredient it can either be rejected or the necessary adjustments to production can be made knowing that typical settings are not going to work. Many pet food manufacturers measure the water activity of their end product, but the idea of using water activity to screen incoming ingredients may be a new, but potentially useful concept.
The most important Specification
For pet food, setting an ideal water activity specification is a critical step in formulating for safety and quality. The specification can be set to avoid microbial proliferation, chemical reactions, physical and structural degradation, and moisture migration. The ideal value can be determined based on the most likely mode of failure, such as texture loss for dry products, chemical degradation for semi-moist products, and microbial growth for wet pet food. Once the ideal water activity is determined, a combination of processing and formulation can be used to achieve that ideal water activity.
The most common processing steps used to produce product that meets water activity specification is to remove moisture through cooking or drying. However, pet food is typically sold on a weight basis, so removing water also reduces the weight of the product and results in lost revenue. Formulation can maximize the amount of moisture in pet food at the water activity specification through the addition of humectants that lower water activity such as sugar, salt, and glycerin.
In addition, the careful monitoring of the water activity of product on the production line will eliminate unnecessary energy waste and weight loss due to processing to lower than ideal water activities, which will maximize revenue. In summary, establishing an ideal water activity specification, formulating to meet that specification, and monitoring production with frequent water activity testing will ensure a safe, quality product with an optimal shelf life and maximum revenue. In short, water activity is the most important pet food specification.
References
- . Phillips-Donaldson, D. 2019. Global pet food sales hit $91 billion in 2018. Pet Food Industry. Retrieved April 18, 2019 from
https://www. petfoodindustry. com/articles/7899-global-pet-food-sales- hit-91 -billion-in-2018
- US Food and Drug Administration. 2019. Compliance Dates. Retrieved April 18, 2019 from
https://www. fda. gov/Food/GuidanceRegulation/FSMA/ ucm540944. htm#AnimalFood
- HARPC. com. 2019. What is HARPC? Retrieved April 18, 2019 from https://www. harpc. com/what-is-harpc/
- US Food and Drug Administration. 2017. Food Code. Pages 22-24. Retrieved April 18, 2019 from
https://www. fda. gov/downloads/Food/GuidanceRegulation/ RetailFoodProtection/FoodCode/UCM595140. pdf –
- Beuchat, L. 1983. Influence of water activity on growth, metabolic activities and survival of yeasts and molds. Journal of Food Protection 46(2):135-141 .
- Scott, W. 1957. Water relations of food spoilage microorganisms. Advances in Food Research 7:83-127.
- Carter, B. P., Syamaladevi, R. M., Galloway, M. T., Campbell, G. S., & Sablani,
- S. S. 2017. A Hygrothermal Time Model to Predict Shelf Life of Infant Formula. In U. Klinkesorn (Ed.), Proceedings for the 8th Shelf Life International
Meeting (pp. 40–45). Bangkok, Thailand: Kasetsart University.
- Eyring, H. 1936. Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J. Chem. Phys. 4:283.
- Katz, E. E. and Labuza, T. P. 1981 . Effect of water activity on the sensory
crispness and mechanical deformation of snack food products. Journal of Food Science 46, 403.
- Carter, B. P., Galloway, M. T., Campbell, G. S., and Carter, A. H. 2015. The critical water activity from dynamic dewpoint isotherms as an indicator of
crispness in low moisture cookies. Journal of Food Measurement and Characterization 9(3):463-470.
- . Labuza, T. P. and Altunakar, B. 2007. Diffusion and sorption kinetics of water in foods, pp. 215-239 in Water Activity in Foods, edited by G. Barbosa-
Canovas, A. J. Fontana, S. J. Schmidt and T. P. Labuza. Blackwell Publishing and IFT, Ames, Iowa.